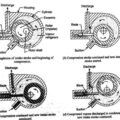
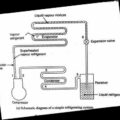
Humidification and Dehumidification
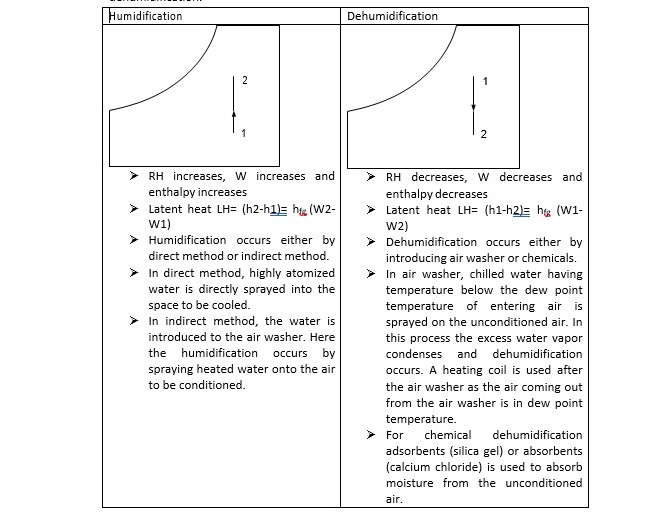
The addition of moisture to the air without change in its DBT is known as humidification and removal of moisture from the air without change in its DBT is called as dehumidification.
- Sensible heat factor:
It is the ratio of sensible heat to total heat.
SHF=
Cooling and Dehumidification
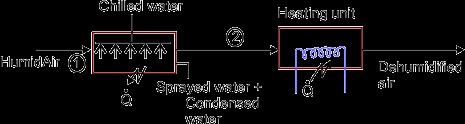
When moist air is cooled below its dew-point by bringing it in contact with a cold surface, some of the water vapor in the air condenses and leaves the air stream as liquid, as a result both the temperature and humidity ratio of air decreases. The effective temperature of cold coil is less than the dew point temperature of entering air. The effective surface temperature of coil is known as apparatus dew point (ADP). This process undergoes in a typical summer air conditioning system. In some commercial air-conditioning plants, chilled water (below the dew point temperature of the mixture) is sprayed into the air to be dehumidified. Then the air leaves with less humidity at the temperature of the chilled water. Next the air is heated to the desired temperature.
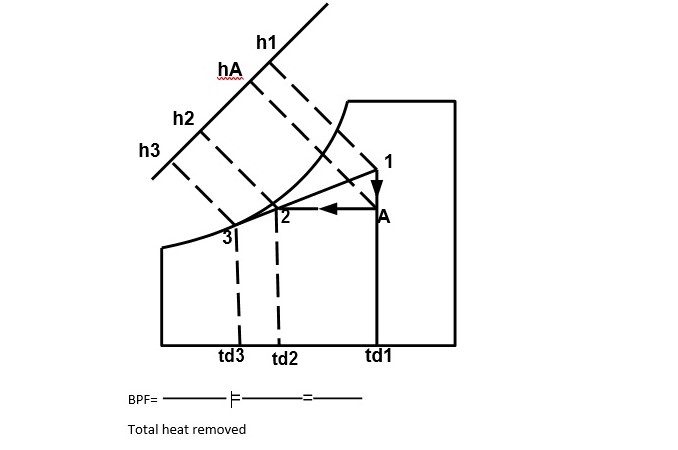
Total heat removed
Q=h1-h2= (h1-hA) +(hA-h2) =LH+SH SHF=
Cooling capacity = ma(h1-h2)
Efficiency = 1- BPF =
Cooling and humidification (Evaporative cooling)
During this process, the air temperature drops and its humidity increases. This can be achieved by spraying cool water in the air stream. The temperature of water should be lower than the dry-bulb temperature of air but higher than its dew-point temperature to avoid condensation. It can be seen that during this process there is sensible heat transfer from air to water and latent heat transfer from water to air. Hence, the total heat transfer depends upon the water temperature. If the temperature of the water sprayed is equal to the wetbulb temperature of air, then the net transfer rate will be zero as the sensible heat transfer from air to water will be equal to latent heat transfer from water to air. If the water temperature is greater than WBT, then there will be a net heat transfer from water to air. If the water temperature is less than WBT, then the net heat transfer will be from air to water. Under a special case when the spray water is entirely recirculated and is neither heated nor cooled, the system is perfectly insulated and the make-up water is supplied at WBT, then at steady-state, the air undergoes an adiabatic saturation process, during which its WBT remains constant.
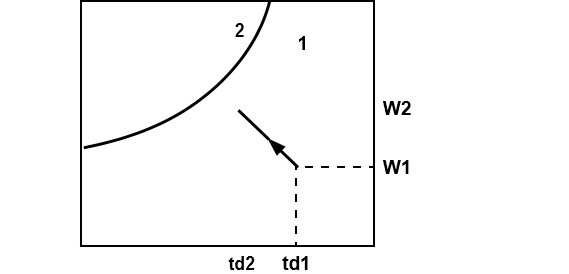
Where mw= mass of water added Ma=mass of dry air
Hw= enthalpy of water
For heat balance
h2= h1 +
hw =h1 + (w2-w1) hw
(w2-w1) hw is neglected as it is very small. So the process is a constant enthalpy process.
Heating and dehumidification
This process can be achieved by using a hygroscopic material, which absorbs or adsorbs the water vapor from the moisture. If this process is thermally isolated, then the enthalpy of air remains constant, as a result the temperature of air increases as its moisture content decreases. This hygroscopic material can be a solid or a liquid. In general, the absorption of water by the hygroscopic material is an exothermic reaction, as a result heat is released during this process, which is transferred to air and the enthalpy of air increases.
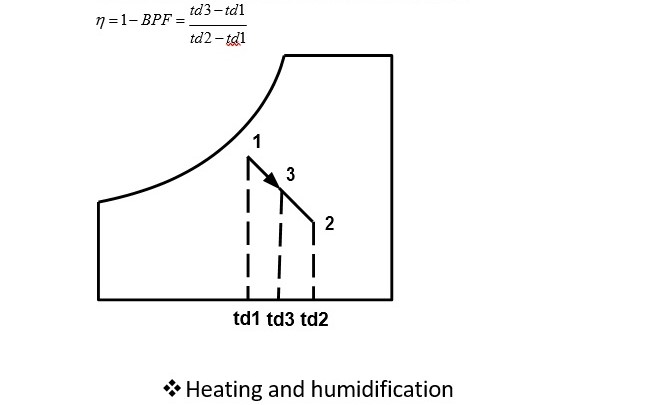
Heating and humidification
This process is used in winter air conditioning system. This is normally done by first sensibly heating the air and then adding water vapour to the air stream through steam nozzles. The air is passed through humidifier having spray water temperature higher than the DBT of entering air. The heat of vaporization of water is absorbed from the spray water and it got cooled.
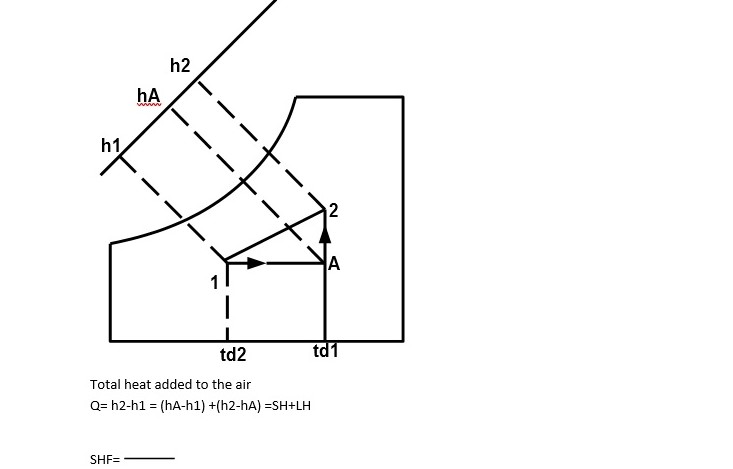
Total heat added to the air
Q= h2-h1 = (hA-h1) +(h2-hA) =SH+LH SHF=
Q1. 100 m3 of air per minute at 35°C DBT and 60% RH is cooled to 20°C DBT passing through a
cooling coil. Find the following
- Capacity of cooling coil in kJ/hr
- Amount of water vapour removed per hour
- RH of air coming out and its WBT
Ans: Given
Pb= atmospheric pressure =1.0132 bar At 35 degree DBT and 60%RH
Ps=0.05628 bar (from steam table at 35°C dbt)
f =Pv/Ps
Pv1= 0.6 *0.05628 =0.033768 bar
Tdp corresponding to pv1 = 26.25°C (from steam table)
Find W1 = 0.622
0.0214 kg/kg of dry air
Find h1= 1.022*td1+ W1 (hfgdp +2.3 t dp) = 89.27 kj/kg
hfgdp (from steam table at dew point temperature of entering air) = 2440 KJ/Kg Air is saturated as leaving air DBT is less than tdp of entering air
So at point 2 wbt=dbt=dpt= 20 °C
Ps2=Pv2 =0.0234 bar (from steam table corresponding to 20°C) Find W2 and h2
W2== 0.622
0.0147 kg/kg of dry air
h2= 1.022*td2+ W2 (hfgdp +2.3 t dp2) =57.19 kj/kg
hfgdp (from steam table at dew point temperature of leaving air) = 2454 KJ/Kg PaVa=Ma*R*td
Ma= = 110.8 kg/min
Capacity of cooling Q= ma (h1-h2) = 3554.46 kj/min =3554.46/210= 16.92 TR Amount of water vapour removed Q= ma(W1-W2) =0.74 kg/min
Alternative way
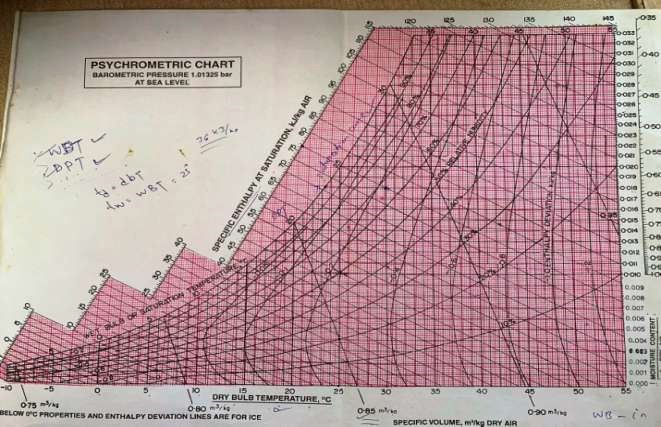
From psychrometeric chart At point 1
35°C dbt and 60% RH W1=0.0214 kg/kg of dry air h1=89.9 kj/kg
tdp= 26°C
Air is saturated as air leaving the coil has lower DBT than dew point temperature. Thus, humidification occurred.
So, tdp=twb=tdp at point 2 as air is saturated. At point 2
W2= 0.0148 kg/kg of air h2= 57 kj/kg